Fly Me O2 The Moon – Features
Mark Symes and Beth Lomax explain the thinking behind how to make oxygen on the Moon
When Gene Cernan climbed the ladder into the Lunar Module of Apollo 17 on 14 December 1972, he became the last human to have set foot on the lunar surface. At the time, few would have believed that nearly 50 years later no further crewed missions to the Moon would have been undertaken. NASA’s Artemis Program aims to change this, by returning astronauts to the Moon as early as 2024. The ambition does not end there though: government agencies and commercial players from around the globe plan to establish a base on the Moon in the coming decade and use this as a testing ground for developing technologies to support sending astronauts to Mars.
One of the key enabling strategies that will allow astronauts to live on the Moon (or Mars) for extended lengths of time is known as In-situ Resource Utilisation (ISRU), the extra-terrestrial equivalent of “living off the land”. Such a strategy would entail astronauts obtaining most of the things necessary to sustain life from what they could find (or make) on the Moon, rather than having to bring these resources with them from Earth. This would have the dual advantages of significantly reducing the payloads that had to be launched from Earth (and so making these launches cheaper and less technically challenging) as well as equipping the astronauts with the ability to fend for themselves independently of resupply from Earth. There is growing evidence that water (in the form of ice) is available in polar regions on the Moon; conversely, foodstuffs could be grown in closed systems using enriched lunar soil (2019 saw the first plants sprout on the Moon in the Chang’e 4 Lunar biosphere). Solar power can be harvested on the lunar surface to provide an energy supply using existing technology. However, one basic commodity that we normally take for granted on Earth is somewhat trickier to obtain on the Moon: oxygen. This will be required at scale, not only for breathing, but also as an oxidising propellant for return to Earth and/or onward missions into deep space. Recent research that we have undertaken suggests that both oxygen and potential materials for manufacturing could be sourced in-situ from the most abundant resource on the Moon – moonrock itself.
Everybody knows the Moon is made of cheese
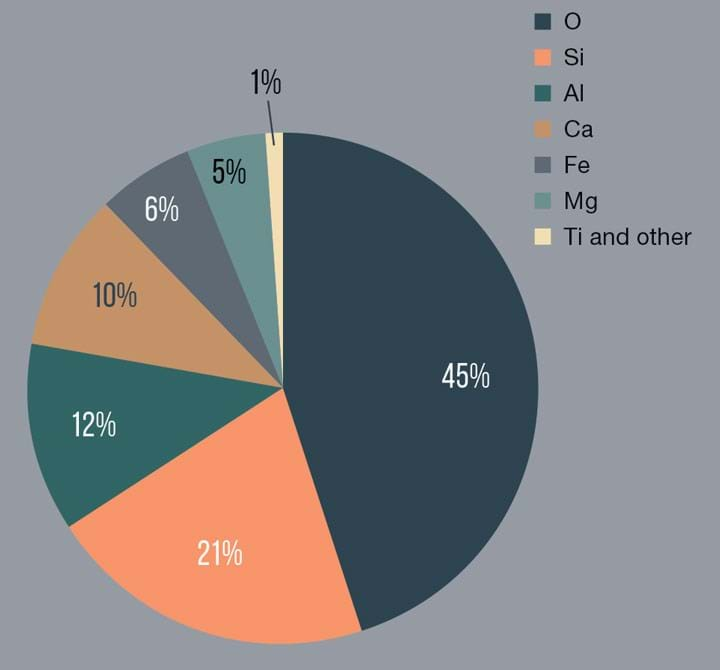
The return to Earth of samples of the lunar surface (also known as lunar regolith) by the Apollo missions has given geologists good insight into the composition of the Moon’s surface. Whilst lunar geology is perhaps not quite as homogeneous as suggested by Wallace and Gromit, it is (at least in comparison to Earth) relatively simple. Lunar regolith consists mostly of oxides of silicon, aluminium, calcium, magnesium and iron, sometimes also with appreciable amounts of titanium oxides. A breakdown of the approximate elemental composition of lunar regolith is shown in Figure 1.
Figure 1: Chart showing the overall average elemental composition (wt%) of lunar regolith
The precise composition at any given point on the Moon’s surface will vary, but by far the most common element present in all classes of lunar regolith is oxygen, at around 45% by weight. This makes regolith a valuable and essentially inexhaustible potential source of oxygen that is available everywhere on the Moon’s surface. This fact has not been lost on engineers with an interest in ISRU on the Moon, but harvesting this oxygen is not easy, as the oxygen is tightly bound within the various metal oxides in the lunar regolith. A number of processes to try and extract oxygen from regolith have been piloted on Earth, including heating regolith-like materials with hydrogen (at 900°C) or methane (at 1,600°C), or melting the regolith-like materials (at 1,600°C or higher) and then electrolysing the molten rock. However, these processes tend to be rather complex and energy inefficient and also tend to give rather low yields of oxygen relative to the amount of regolith used (ie most of the oxygen in the regolith is not extracted).
A simpler process that could extract oxygen from regolith without the need to melt the regolith and without the need for additional hydrogen or methane would suit the requirements of a system able to operate autonomously on the Moon much more closely. In the following, we describe the process that we are developing as part of a collaboration between the University of Glasgow, Metalysis, and the European Space Agency that allows nearly all of the oxygen present in a given sample of regolith to be extracted, without the need to melt the regolith. A valuable byproduct of this process is the formation of metallic alloys that could be used for manufacturing on the lunar surface, thus going some way towards giving lunar outposts the ability to survive without constant resupply from Earth. The process is based on electrochemical
technology originally developed at the University of Cambridge (and subsequently developed by Metalysis), known as the Metalysis FFC Cambridge process.
The FFC Cambridge process
The FFC Cambridge process (named after its inventors, Fray, Farthing and Chen, and their institution at the time of discovery) is an electrochemical method for the reduction of metal oxides to the corresponding metals that works with metal oxides in the solid (ie not molten) state. A diagram illustrating how this process works is given in Figure 2. The metal oxide to be reduced is used as a cathode: electrons will enter this electrode and reduce the metal ions in the oxide down to the metal in oxidation state zero, according to the equation shown on the left-hand side of Figure 2. As this happens, oxide anions (O2–) leave the cathode and migrate across the molten calcium chloride electrolyte to the anode. The calcium chloride electrolyte must be kept at temperatures above 800°C in order to be conductive enough to allow these anions to move across the cell (and so to complete the electrochemical circuit), but this temperature is still significantly below that required to melt lunar regolith. The FFC Cambridge process was originally developed for producing titanium metal from titanium dioxide on Earth, and in that context the potential to produce oxygen gas (O2) as a byproduct of metal oxide reduction was considered unimportant. Traditional Metalysis FFC Cambridge electrochemical cells therefore use a carbon-based anode, with which the O2– anions react to produce carbon dioxide (CO2, see equation on the upper right-hand side of Figure 2). Although this leads to the gradual consumption of the carbon anode, these anodes are cheap and plentiful on Earth, rendering the process economically viable.

Figure 2: A general schematic showing the operating principle of the Metalysis FFC Cambridge process for the reduction of metal oxides at the cathode, and the production of either carbon dioxide (when using a carbon anode) or oxygen (when using an inert anode)
However, for our purposes, CO2 is not an especially useful product. Instead, we needed to adapt the system so that the anode product would be oxygen (lower equation on right hand side of Figure 2), rather than carbon dioxide. This in turn required the use of “inert” anodes, that would not react with the O2– anions, but which would instead facilitate these anions combining to give O2, simultaneously releasing electrons to be used in the reduction of the metal ions in the lunar regolith at the cathode. An anode able to perform this reaction based on tin dioxide (SnO2) was used in our initial study which (although good enough to show proof-of-concept) is not an ideal material for this purpose as we discuss below. Indeed, finding suitable anodes for oxygen generation under these conditions remains one of the major unsolved challenges of this approach.
Electrolysing “Moonrock”
As you might imagine, genuine samples of lunar regolith are rather scarce on Earth. Our experiments to date have therefore focussed on the use of simulated lunar regolith: materials developed in labs on Earth that have similar composition and the look and feel of the real thing. These lunar regolith simulants are themselves rather hard to obtain, so we have been working so far on the gramme scale (although for terrestrial applications such as production of titanium metal, Metalysis has systems able to function using tons of oxide at a time). In a typical experiment, a setup similar to that shown in Figure 3 is used. A basket containing the regolith simulant is submerged in molten calcium chloride, along with an inert anode and a thermocouple, and the entire gas-tight cell is heated in an electric furnace to keep the electrolyte molten. Current is then passed between the anode and cathode to drive the reduction of the oxide material at the cathode and produce oxygen gas at the anode that can be filtered and analysed in real time.
Figure 3 shows important design features and operational conditions of the process, and highlights where a reactor designed for operation on the Moon would differ from the reactors typically used for lab-based research on Earth. Evidently, a molten salt reactor built to survive and run autonomously on the Moon may look rather different to its terrestrial counterpart. Significant research efforts are currently under way at ESA’s European Space Research and Technology Centre in the Netherlands, Metalysis near Sheffield, the University of Glasgow, and other institutions across Europe, to work on optimising the scientific and engineering parameters for such a lunar reactor.

Figure 3: Illustration of our Metalysis FFC Cambridge cell for simultaneous reduction of lunar regolith simulant and production of oxygen
When all the regolith has been reduced (and hence all the oxygen extracted), a metallic alloy is recovered (see Figure 4). The composition of this alloy is rather complex, which is not unexpected given that it is produced by the direct reduction of complex natural materials. However, we have been able to show that distinct alloy groupings tend to form consistently. Determining whether or not these alloys will prove useful on the Moon for any specific purpose, or whether perhaps they might be suitable as low-grade materials for additive manufacturing or rocket launch pads will require scaleup of the system so that larger volumes of these reduced alloys can be produced and their mechanical and materials properties fully investigated.
Crucially, we have demonstrated that it is possible to remove >95% of all the oxygen present in the original regolith simulant by this method, which in a lunar context would equate to around 40-45 kg of oxygen for every 100 kg of regolith. 100 kg of regolith has a volume of approximately 65 L, and NASA estimates that an astronaut needs to breathe just under 0.9 kg of oxygen per day. That means that using the Metalysis FFC Cambridge process, enough oxygen to allow one astronaut to breathe for 24 hours could be obtained from only 1.5 L of regolith. These numbers look entirely feasible (especially if one considers that this oxygen would be metabolised to carbon dioxide and that chemical processes exist for recycling the oxygen from this carbon dioxide for the astronauts to breathe over and over again). Indeed, this level of oxygen yield from regolith also opens up the possibility that the Metalysis FFC Cambridge process could be used to generate oxygen on the Moon in sufficient quantities to allow rockets returning to Earth (or travelling further afield, perhaps to Mars) to fill up on the Moon with oxygen to use in their propulsion systems. This would save having to bring this rocket oxidant from Earth and would make human exploration of the solar system a much more achievable prospect.

Figure 4: Lunar regolith simulant before electrolysis (left) and after reduction by the Metalysis FFC Cambridge process to metallic alloys (right)
Future challenges
Of course, many challenges remain before a large-scale oxygen production plant is functioning on the lunar surface! Reliable operation and infrequent maintenance will be essential in this context. In the short term, inert anodes that are mechanically and electrochemically stable need to be developed further. This is no small challenge, given that such anodes have been considered a holy grail of the terrestrial aluminium industry for decades, yet still remain elusive. However, a critical difference does exist here inasmuch as anodes for electrochemical aluminium extraction on Earth must be relatively inexpensive in order to be economically viable; such restraints on material choice are much less important when designing systems for applications in space. Ideal materials for the reactor housing and reactor components also need to be identified for the high temperature, oxygen-rich and salty environment, to maximise reactor lifetime and minimise any oxygen lost via corrosion. In the longer term, a scaled-up cell will then need to be designed that is compact and robust enough to be sent to the Moon, and that will be able to work effectively on the lunar surface under the conditions prevailing there (near vacuum, large temperature swings, low gravity). All of these factors could have a major influence on the operation of the system and present major engineering challenges. For example, how will the lower gravity on the Moon affect the behaviour of the oxygen bubbles as they form in the calcium chloride melt? Will it be easier or harder for them to be harvested? How will the near-vacuum on the lunar surface affect the rate of evaporation of the calcium chloride from the molten electrolyte? What kind of electrochemical cell designs can be developed that would allow for continuous production of oxygen? And will regolith need to be shovelled into the cells periodically by a human operator, or could an automated system be devised? These are just a few of the challenges that remain and that the European Space Agency hopes to tackle over the next 3-10 years. If we can show that our system can overcome these challenges and that operation in the lunar environment could be feasible, then this technology could form a cornerstone of the goal of establishing a sustainable human presence on the lunar surface as a springboard to voyaging to Mars and beyond. Whatever the future may hold for this technology, it is clear that chemical engineering has as significant a role to play in exploration of the solar system as it does on Earth.


Comments are closed.