Leading spinal researcher develops new tissue regeneration approaches for back pain
Karin Wuertz-Kozak described her lab test equipment as a gym for cells.
Stretching and compressions tests using bioreactors—her lab equipment—can make a difference in understanding how cells respond to mechanical cues and how that affects disease progression, specifically for spinal disc degeneration, common to millions of Americans.
Wuertz-Kozak, a faculty researcher at Rochester Institute of Technology, is contributing expertise in biomedical engineering and pharmacology to regional and national collaborations toward solving disc degeneration. It is an expensive condition that results in lost mobility and worktime as well as an economic burden in the US of nearly $134 billion, according to the Institute for Health Metrics and Evaluation.
High physical activity or obesity contributes to back pain and spinal degeneration. What is not so well known is exactly how mechanical signals produce changes in cells that lead to degeneration. Wuertz-Kozak is exploring this aspect, specifically the link between mechanical loading, disc degeneration, inflammation in the disc, and back pain.
Provided by K. Wuertz-Kozak
Spinal disc degeneration and back pain is a condition that results in lost mobility and worktime for individuals, as well as an economic burden in the US in the millions.
“We have bio-reactors in our lab that simulate mechanical loads on the body, but in a scaled-down version. The bioreactor used can compress and stretch cells. It sounds a little bit medieval,” said Wuertz-Kozak, the Kate Gleason Endowed Professor in RIT’s Department of Biomedical Engineering. She brings an eclectic background to her work with experience in pharmacology, biomechanics, biomedical engineering and biology. Her clinical collaborations are world-wide, spanning locally with the University of Rochester to Fukushima Medical University in Japan.
Receiving nearly $2 million in funding since she came to RIT three years ago, and building upon career funding and research prior to arriving, her goals are to better understand disc degeneration at the cellular level and to develop novel, non-invasive solutions for spinal care .
Her work on the role and effects of mechanical loading in the context of back pain builds on previous research funded by a grant from the Swiss National Science Foundation in which she studied transient receptor potential (TRP) channels in the disc. The work now continues with a recent award through the National Institutes of Health to fund an investigation of the transient receptor potential channels V4 (TRPV4) in intervertebral disc mechanotransduction— the cells’ ability to sense stimuli and convert signals to biological processes.
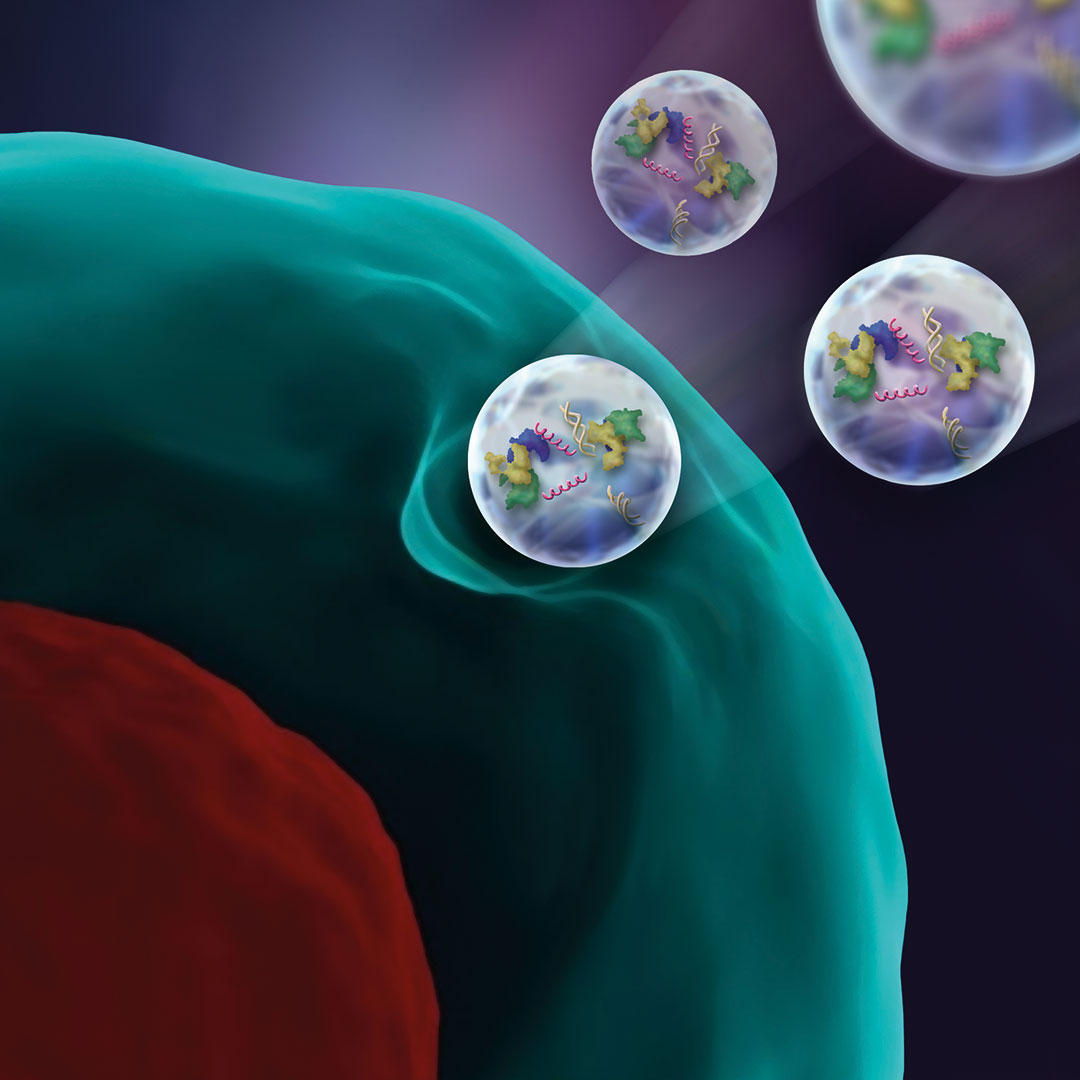
Provided by K. Wuertz-Kozak
Extracellular vesicles are derived from stem cells and are a promising option to help regenerate damaged spinal disc cells.
“Mechanical loading is not just stretching due to physical activity, there are different mechanical aspects that the cells will sense,” she said. “Changes in the stiffness or topography of the extracellular matrix, which happen during degeneration and aging, will also constitute a mechanical signal, and might alter the cellular response to physical activity.
“Our stretching and compression bioreactors help us to understand how cells respond to mechanical cues and how that affects disease progression. In the past, we were able to show that stretching of cells at high magnitudes leads to inflammation in the disc, which is suspected to be a main contributor to disc-related back pain.”
Understanding sources of chronic inflammation can give clues to relieving disc-related back pain and is a crucial part of developing novel, molecular treatment options for patients. One promising approach to modulate and control tissue inflammation and induce regeneration is through stem cells. Although stem cells have proven successful in the regeneration of many tissues, the intervertebral disc constitutes a drastically harsh cell environment, Wuertz-Kozak explained. An alternative is exploring extracellular vesicles (EVs) derived from stem cells—bioparticles that carry proteins, lipids, DNA, and various types of RNA.
“Stem cell-derived EVs are thought to contain many of the regenerative goodies that stem cells produce and may thus have great regenerative potential. We currently have two very exciting projects on stem cell derived EVs in the lab, both of which use the CRISPR technology,” she said. “The CRISPR-Cas9 system has generated a lot of excitement in the scientific community as it is a fast, comparatively cheap and very accurate and efficient technology to add, remove, or alter genetic material.”
In one of the projects, Wuertz-Kozak and RIT colleague Thomas Gaborski, professor of biomedical engineering, are collaborating with Zhen Ma, associate professor of engineering at Syracuse University on developing a biomanufacturing process to increase the quantity of EVs produced by stem cells. Cross-disciplinary in nature, the project team also includes Aslan Dehghani ’21 Ph.D. (microsystems engineering), a lead EV scientist and bioengineer at Sartorius, at an international bio-separation firm.
“This project is a perfect example of how collaborative the work environment in the biomedical engineering department is,” says Wuertz-Kozak, who leads the Tissue Regeneration and Mechanobiology Lab in RIT’s Kate Gleason College of Engineering. The lab focuses on cell and molecular biology, with applications to improve patient care, to understand disease better and to offer new treatment options.



Comments are closed.