Researchers report advances toward room-temperature fluoride-ion batteries
Fluoride-based rechargeable batteries could offer very high energy density. However, current fluoride batteries use molten salt electrolytes and therefore must be operated at high temperatures. A team of researchers from Caltech, the Jet Propulsion Laboratory (managed by Caltech for NASA), the Honda Research Institute, and the Lawrence Berkeley National Laboratory now report two advances that could lead the way to room temperature fluoride batteries. Her article is published in Science.
Fluoride ion batteries offer a promising new battery chemistry with up to ten times higher energy density than currently available lithium batteries. Unlike Li-ion batteries, FIBs do not pose a safety risk due to overheating, and sourcing the raw materials for FIBs results in a significantly lower environmental impact than the lithium and cobalt extraction process.
-DR. Christopher Brooks, Chief Scientist, Honda Research Institute, co-author
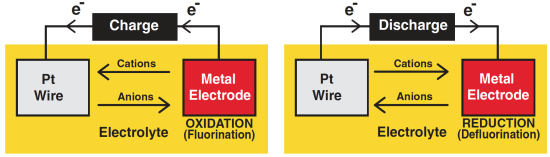
Scheme of the external electron flow, the electrolyte ion shuttle and the redox reactions that occur on fluoride ion battery (FIB) electrodes during charge or discharge cycles. Davis et al.
The first advance is the development of a liquid electrolyte at room temperature based on a stable tetraalkylammonium salt-fluorinated ether combination. The second is a copper-lanthanum trifluoride core-shell cathode material that exhibits reversible partial fluorination and defluorination reactions.
Fluoride ion batteries are potential next generation electrochemical storage devices that offer high energy density. Such batteries are currently restricted to operation at high temperatures, since suitable electrolytes which conduct fluoride ions are only known in the solid state.
We report on a liquid fluoride ion conducting electrolyte with high ionic conductivity, wide operating voltage and robust chemical stability based on dry tetraalkylammonium fluoride salts in ether solvents. In combination with this liquid electrolyte with a copper-lanthanum trifluoride (Cu @ LaF3) core-shell cathode, we show reversible fluorination and defluorination reactions in an electrochemical fluoride ion cell cycled at room temperature. Fluoride-ion-mediated electrochemistry offers a way to develop capabilities beyond lithium-ion technology.
– Davis et al.
The researchers have received two US patents.
Co-author Robert Grubbs, Victor and Elizabeth Atkins Professor of Chemistry at Caltech and 2005 Nobel Prize Winner in Chemistry said:
Fluoride batteries can have higher energy density, which means they can last longer – up to eight times longer than batteries in use today. However, working with fluoride can be difficult, especially because it’s so caustic and reactive.
While lithium ions are positive (called cations), the fluoride ions used in the new study carry a negative charge (and are called anions). Working with anions in batteries presents both challenges and benefits.
For a battery that lasts longer, you need to move a greater number of charges. Moving multiply charged metal cations is difficult, but a similar result can be achieved by moving several singly charged anions that move comparatively easily. The challenges with this scheme are that the system operates at usable voltages. In this new study, we show that anions actually deserve attention in battery science, as we show that fluoride can work at sufficiently high voltages.
– Simon Jones, chemist at JPL and corresponding author of the new study
The key to making the fluoride batteries operate in a liquid rather than a solid state is an electrolyte liquid known as bis (2,2,2-trifluoroethyl) ether, or BTFE. This solvent helps keep the fluoride ion stable so it can transport electrons back and forth in the battery.
BTFE is made up of several chemical groups arranged in such a way that the molecule has two positively charged regions that interact strongly with fluoride as opposites attract. Simulations showed how these charged regions cause BTFE molecules to surround fluoride and dissolve it at room temperature.
The next step in refreshing fluoride-based batteries is to extend the life of the cathode and anode. The team has already made progress by stabilizing the copper cathode so that it does not dissolve in the electrolyte.
Battery tests are in progress. The work was supported by the Resnick Sustainability Institute and the Molecular Materials Research Center, both from Caltech, the National Science Foundation, the Department of Energy Office of Science, and the Honda Research Institute.
resources
-
Victoria K. Davis, Christopher M. Bates, Kaoru Omichi, Brett M. Savoie, Nebojša Momčilović, Qingmin Xu, William J. Wolf, Michael A. Webb, Keith J. Billings, Nam Hawn Chou, Selim Alayoglu, Ryan K. McKenney Isabelle M. Darolles, Nanditha G. Nair, Adrian Hightower, Daniel Rosenberg, Musahid Ahmed, Christopher J. Brooks, Thomas F. Miller III, Robert H. Grubbs, Simon C. Jones (2018) Fluoride electrodes: Liquid electrolytes for high-energy fluoride ion cells ” Science Vol. 362, Edition 6419, pp. 1144-1148 doi: 10.1126 / science.aat7070


Comments are closed.