Using bioelectrohydrogenesis left-over residues as a future potential fertilizer for soil amendment
Electrohydrogenesis effluent as a potential biofertilizer
To characterize the electrohydrogenesis left-over residues as potential biofertilizers, the sample from the operating reactors was performed a 16S rRNA sequencing test, and interestingly, the results revealed that the bio-electrohydrogenesis effluent was enriched with various microorganisms including plant growth-promoting microbes that display biofertilizer-like features. Among the well-known plant-promoting bacterial genera observed in DF-MEC residues included Azospirillum, Mycobacterium, Chryseobacterium, Paenibacillus, Rhizobacter, Pseudomonas, Achromobacter, Bradyrhizobium, Actinomyces, Sphingomonas, Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium, Gordonia, Rhodococcus, Bacillus , Methylobacterium-Methylorubrum, Microbacterium, Flavobacterium, Devosia, Acinetobacter, Mesorhizobium, Enterobacter, Aeromonas, Beijerinckia, etc.24,25,26 (Fig. 2). Lots of investigations working on the feasibility of using biofertilizers other than chemical fertilizers have revealed that those aforementioned microbes play a major role in providing the required nutrients for enhanced crop yield.
Figure 2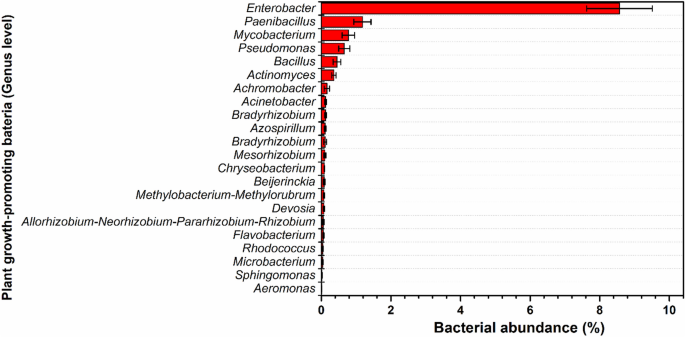
The abundance of the Plant growth-promoting bacteria (genus level) detected from the DF-MEC digestate (%).
Nitrogen-fixing microorganisms
The detected nitrogen-fixing microorganisms from the electrohydrogenesis effluent include Azospirillum sp. (0.11 ± 0.02%), rhizobia (Rhizobium (0.058 ± 0.02%), Bradyrhizobium (0.11 ± 0.04%), and Mesorhizobium (0.1 ± 0.03%)), and Beijerinckia (0.08 ± 0.03%) (Fig. 2) and were repeatedly reported for their superior contribution to the plants’ nitrogen requirements through biological nitrogen fixation, which is an important component of sustainable agriculture25. Although the atmosphere counts about 78% N2, it couldn’t be used by plants in its natural state. Prior to getting used by plants, it needs to be converted to ammonia, which is the readily assimilable form of nitrogen by plants/or crops via a biological nitrogen fixation mechanism25. The biological nitrogen fixation mechanism is summarized in Fig. 3.
Figure 3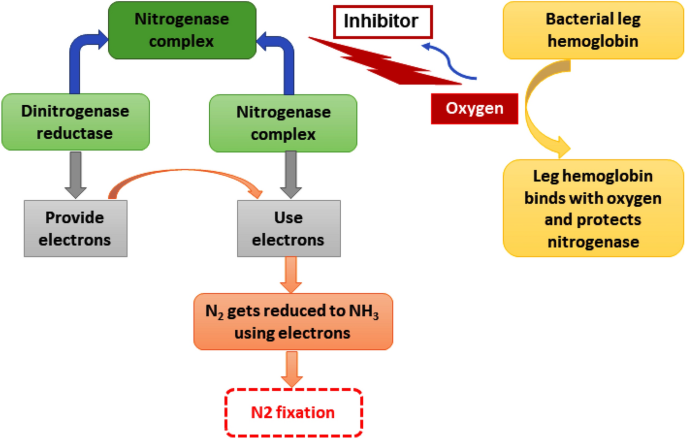
Mechanism of nitrogen fixation bio-catalyzed by nitrogenase enzyme. The plant growth-promoting bacteria produce nitrogenase which is a complex enzyme consisting of dinitrogenase reductase and dinitrogenase. This complex enzyme plays a major role in molecular N2 fixation. Dinitrogenase reductase provides electrons and dinitrogenase uses those electrons to reduce N2 to NH3. However, oxygen is a potential threat to this process since it has the ability to get bound to the enzyme complex and make it inactive and consequently inhibit the process. Interestingly, bacterial leghemoglobin has a strong affinity for O2 and thus gets bound to free oxygen more strongly and effectively to suppress the available oxygen effects on the whole process of nitrogen fixation.
Phosphate-solubilizing microorganisms
Furthermore, various phosphate-solubilizing and mineralizing strains were also found in bioelectrohydrogenesis residues collected from our DF-MEC integrated reactors. Among those microorganisms with the ability to solubilize/metabolize the insoluble inorganic phosphorus, the dominant bacterial genera included Pseudomonas (0.65 ± 0.15%), Bacillus (0.44 ± 0.11%), Rhodococcus (0.04 ± 0.009%), Rhizobium (0.05 ± 0.02% ), Microbacterium sp. (0.04 ± 0.01%), Achromobacter (0.16 ± 0.07%), and Flavobacterium (0.058 ± 0.014%) (Fig. 2). Though enormous amounts of phosphorus are available in the soil, its high portion never contributes to plant growth in its primitive state, unless it is bio-transformed into absorbable forms including monobasic and dibasic. Microbial phosphate solubilizing mechanisms are well described in Fig. 4.
Figure 4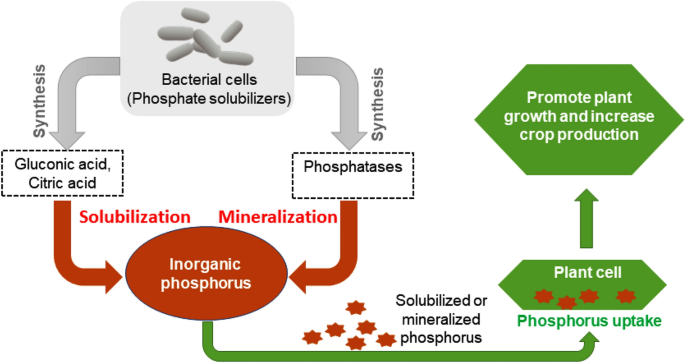
Inorganic phosphorus solubilization by phosphate-solubilizing rhizobacteria. A bacterium solubilizes inorganic phosphorus through the action of low molecular weight organic acids such as gluconic and citric acids. The hydroxyl (OH) and carboxyl (COOH) groups of these acids chelate the cations bound to phosphate and thus convert insoluble phosphorus into a soluble organic form. The mineralization of soluble phosphorus occurs by synthesizing different phosphatases which catalyze the hydrolysis process. When plants incorporate these solubilized and mineralized phosphorus molecules, eventually, overall plant growth and crop yield significantly increase.
Phytohormone-producing microorganisms
In this current work, the electrohydrogenesis effluent also contained bacterial genera such as Mycobacterium (0.77 ± 0.18%), Allorhizobium (0.05 ± 0.02%), Pararhizobium (0.05 ± 0.02%), Paenibacillus (1.18 ± 0.24%), Bradyrhizobium (0.11 ± 0.04%), Rhizobium (0.05 ± 0.02%), Acinetobacter (0.14 ± 0.02%), and Azospirillum (0.11 ± 0.025%) (Fig. 2) that have the ability to synthesize indole-3-acetic acid/indole acetic acid ( IAA) through indole-3-pyruvic acid and indole-3-acetic aldehyde25. IAA is a well-known type of phytohormone that enhances plant/crop growth. Particularly, Azospirillum sp., also produce various phytohormones namely cytokinins, gibberellins, ethylene, abscisic acid and salicylic acid, auxins, vitamins such as niacin, pantothenic acid, and thiamine. The conceptual model delineating the positive effects of inoculation with Azospirillum sp. a phytohormones producer plant growth-promoting rhizobacteria and its detailed functions on plant growth are summarized and illustrated in Fig. S1. Therefore, the existence of those rhizobacteria in the bioelectrohydrogenesis residues further implies the suitability of considering the DF-MEC left-over residues as potential biofertilizers.
Heavy metals-bioremediating microorganisms
Some other bacterial genera with the ability to bioremediate the heavy metal toxicity were also found within the bioelectrohydrogenesis left-over residues as well. Among the detected plant growth-promoting bacterial genera; Rhizobium (0.058 ± 0.023%), Mesorhizobium (0.1 ± 0.026%), Bradyrhizobium (0.11 ± 0.04%), Pseudomonas (0.65 ± 0.15%), and Achromobacter (0.16 ± 0.077%) were reported for their key contribution to alleviate the toxicity of the heavy metals via bioremediation process and improve the soil quality for a relief plant development26 (Fig. 2). Other detected heavy metals-bioremediating microorganisms’ species were Chryseobacterium sp. (0.08 ± 0.007%), Azospirillum (0.11 ± 0.02%), Bacillus (0.44 ± 0.11%), Enterobacter (8.57 ± 0.9%), Gordonia (0.06 ± 0.02%), Paenibacillus (1.18 ± 0.24%), Pseudomonas (0.65 ± 0.15%), and Actinomycetes (0.36 ± 0.05%) that either use microbial siderophores or enzymatic biodegradation process.
Electrohydrogenesis left-over residues as a potential source of essential elements for plant growth
As aforementioned in “Materials and methods” section, the electrohydrogenesis left-over residues contained diverse microbial communities that degraded the MEC substrate and generate biogas and inorganic compounds. Moreover, it has been reported that those inorganic nutrients are generally available in fermentation effluent in readily plant-utilizable formats owing to substrate mineralization27. Besides detecting various plant growth-promoting microorganisms in the electrohydrogenesis effluent, a larger number of mineral elements essential for promoted growth and development of crop plants were also investigated and analyzed from the residues. The detected primary and secondary macro-elements’ concentrations in the residues were arranged in decreasing order as follows P > S > Na > K > N > Ca > Mg. Interestingly the findings show that the residues abundantly contained Phosphorus (2,766 × 103 mg/ L), Nitrogen (274 mg/L), Potassium (282 mg/L), Calcium (17.66 mg/L), Magnesium (16.3 mg/L), Sulfur (1,225 × 103 mg/L), and Sodium (294.3 mg /L) which are well known as macro-nutrients needed in larger amounts for enhanced plant/ crop growth (Fig. 5).
Figure 5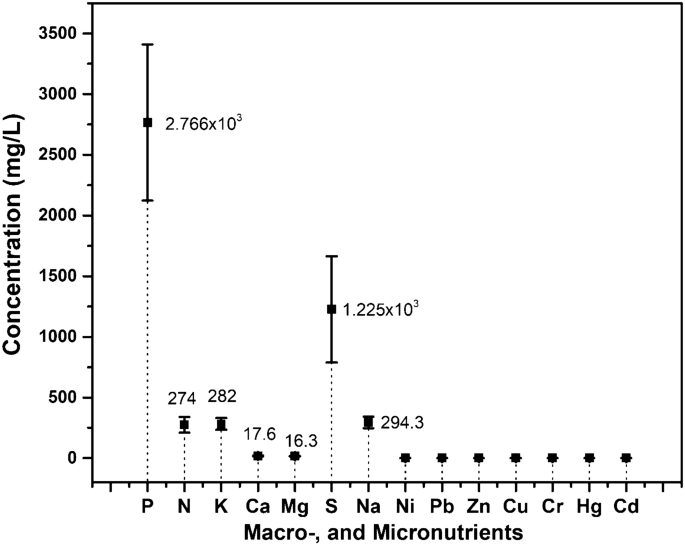
Macro-, and micronutrients detected from the bio-electrohydrogenesis left-over residues (mg/L).
Moreover, small amounts of the microelements including Ni, Pb, Zn, Cu, Cr, Hg, Cd were also found in the electrohydrogenesis residues, and consistently these elements are generally required in small quantities for the development of plants (Fig. 5), otherwise, their high concentrations are toxic for the plant cells thus suppressing or inhibiting plant growth. The detected concentrations for the main microelements in this current research ranged only from 0.36 to 9.6 × 10–5 mg/L and were all reported to play fundamental roles in plant metabolic reactions.
Cultivation of the leguminous crops using electrohydrogenesis left-over residues as fertilizer
After evaluating the plant-growth promoting bacterial communities and the macro- and micronutrients required for plant/crop growth in the electrohydrogenesis left-over residues, the latter was directly used as fertilizer to grow three different plant species including tomato, chilli, and brinjal as afore-described in the “Materials and methods” section. To access the potentials of the electrohydrogenesis effluent as fertilizer, the plants grown in the soil amended with the effluent (Soil + Effluent), were directly compared with their corresponding control plants (Soil + water). The results indicated that at the end of 1st month, the plants with effluent grew faster and generated a good amount of branching than the control plants (see Fig. 6), possibly due to the availability of both microbial species with bio-fertilizing aspects and micro- and macronutrients in the effluent.
Figure 6
Analysis of the plant growth at the end of the 1st month of cultivation. (a) Tomato in soil with effluent, and its control without effluent (b); (c) Chilli grown in soil with effluent, and its control without effluent (d); and (e) brinjal grown in soil with effluent, and its corresponding control grown without effluent (f) (after 2 months).
For instance, tomato (Solanum lycopersicum L.) and chilli (Capsicum annuum L.) height in soil + electrohydrogenesis effluent was ~ 36.9 ± 2.1 cm and ~ 32.6 ± 0.8 cm respectively which was ~ 2.03 and ~ 1.2 times the height of their corresponding plant species in the control protocol, respectively (see Fig. 7). However, the brinjal species (Solanum melongena L.) didn’t show any remarkable height differences in both protocols after a month of cultivation (data not shown), probably due to their low adaptive characteristics to the new environment. However, after the 2nd month, the brinjal height in soil + effluent became 2.7 times that of the brinjal control cultivated without effluent (see Fig. 6e,f). Moreover, both the number of the plants’ leaves and their length in plants cultivated in soil + effluent, were remarkably higher than in plants grown without the supply of the effluent.
Figure 7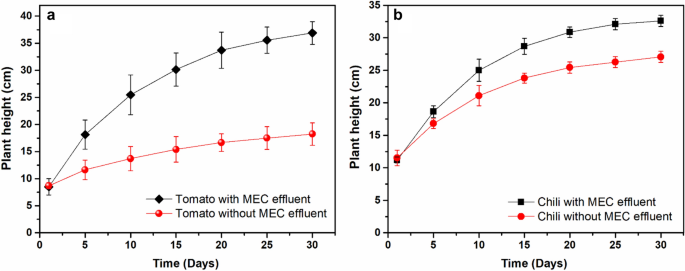
Daily plant growth analysis within one month of cultivation. (a) Tomato growth monitoring, (b) Chili growth analysis.
At the end of the 3rd month, the plants in soil + electrohydrogenesis effluent generated more fruit with big size than the control plants (see Fig. 8), but the tomato (Solanum lycopersicum L.) didn’t generate fruits in both protocols at that time probably due to the high weather temperature that inhibitory affected its continuous growth, as previously reported that tomato species are generally so sensitive to temperature change28,29. The final yield was evaluated in terms of the size and number of fruits per cultivated plant. Chili cultivated in soil with MEC effluent generated 3 fruits/plant and its corresponding control without effluent produced only 1 fruit/plant. The chili fruit size in soil + effluent was 16 cm, approximately 18.7% higher than its corresponding control. Moreover, at the time of collecting data, the brinjal plant cultivated in soil with MEC effluent generated brinjal fruits whereas its corresponding cultivated without electrohydrogenesis effluent started flowering (see Fig. 8). These further indicate the significant contribution of the electrohydrogenesis effluent in speeding up the plant growth. Herein, the electrohydrogenesis left-over residues have notably improved the soil quality and significantly promoted the plants’ phenology characterized by plant growth, the generation of new leaves, flowering, and the production of fruits.
figure 8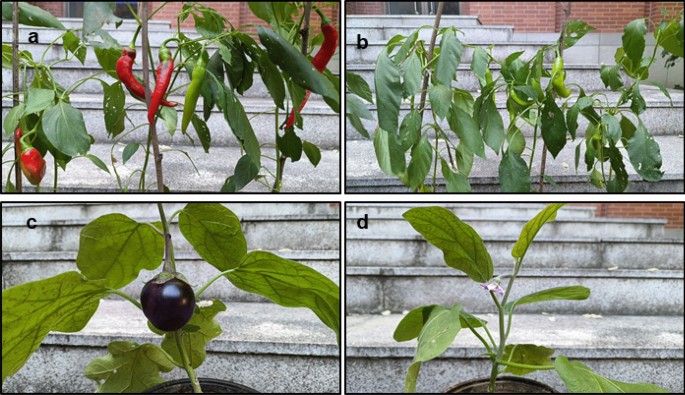
Analysis of plant growth characterized by the flowering and fruiting process at the end of the 3 months. (a) Chili grown in soil with effluent, and its control without effluent (b); (c) brinjal grown in soil with effluent and its corresponding control grown without effluent (d).


Comments are closed.